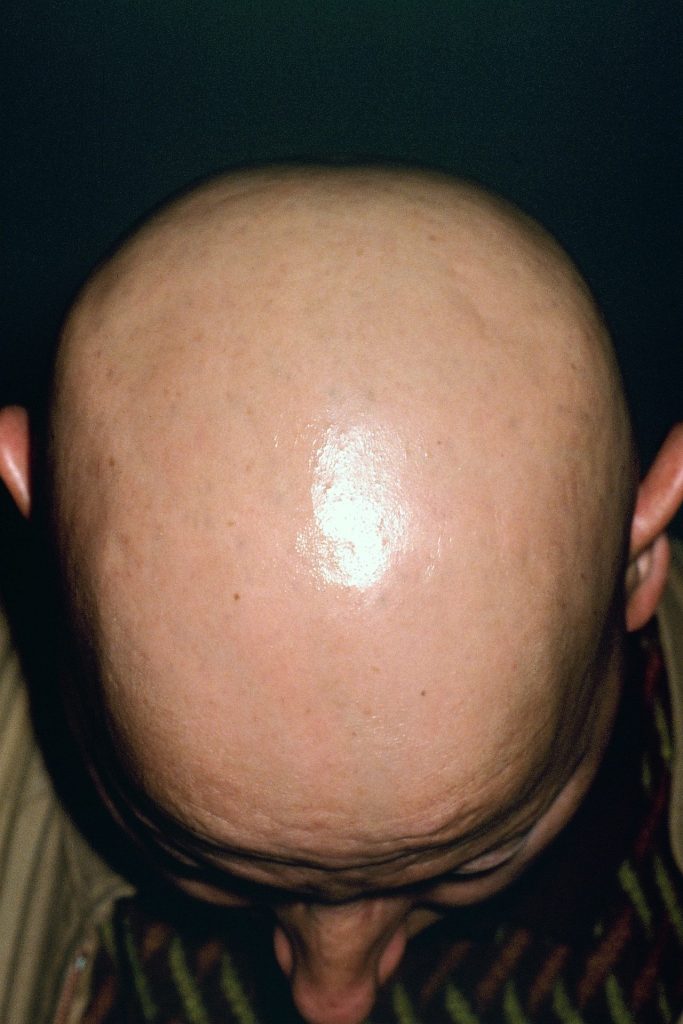
The last few months has brought national attention to the hair loss condition known as alopecia and there is now some very good news from those who suffer from it. According to recent reports, the U.S. Food and Drug Administration has officially approved a brand new drug for treatment of alopecia following its impressive success rate.
@People reports, in a formal announcement, the FDA has decided to approve a new drug called baricitinib, which is used to treat alopecia and restore hair growth. The oral tablet, made by pharmaceutical company Eli Lilly, is specifically intended to be used for those who suffer from alopecia areata—a skin disease that impacts over 6 million Americans and is known as the second most common form of hair loss.
Baricitinib was previously approved by the FDA for the treatment of rheumatoid arthritis back in 2018, however once it was used on alopecia sufferers, patients saw 80% of hair regrowth in 36 weeks in comparison to just 5% of regrowth from those who took a placebo pill.
Speaking about the latest effort to treat alopecia, Dr. Kendall Marcus, Director of Division of Dermatology and Dentistry in the FDA’s drug evaluation and research program, had this to say:
“Access to safe and effective treatment options is crucial for the significant number of Americans affected by severe alopecia. Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata.”
Additionally, late last month Concert Pharmaceuticals released its own hair loss treatment drug. The product reportedly had a high success rate during clinical trials, with patients said to have regained at least 80% of their hair in a 24-week period.
Want updates directly in your text inbox? Hit us up at 917-722-8057 or click here to join!
















